Topic: Radioactive Decay Data
Radioactive Decay Data
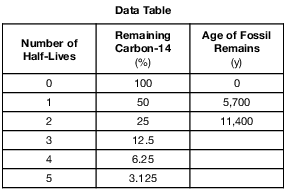
The table below shows the radioactive decay of carbon-14. Part of the table has been left blank.
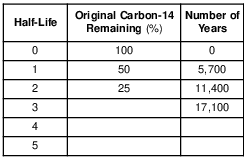
After 22,800 years, approximately what percentage of the original carbon-14 remains?
(1) 15%
(2) 12.5%
(3) 6.25%
(4) 3.125%
The graph below represents the decay of a radioactive isotope into a stable disintegration product.

Which remains could be dated using this radioactive isotope?
(1) seeds from the earliest grasses
(2) mastodont bone
(3) feathers from the earliest birds
(4) Naples tree trunk

Which radioactive isotope has a half-life closest to the half-life of the radioactive isotope rubidium-87?
(1) iodine-131
(2) gold-198
(3) beryllium-10
(4) lutetium-176
A bar graph of the radioactive decay of carbon-14 is shown below.
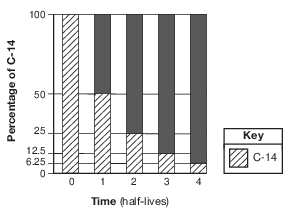
The solid black sections of the bars on the graph represent the percentages of
(1) carbon-14 from the original sample that has not decayed
(2) uranium-238 from the original sample that has not decayed
(3) nitrogen-14 decay product resulting from the radioactive decay
(4) lead-206 decay product resulting from the radioactive decay
A fossil formed 11,400 years ago. Which percentage of the original amount of carbon-14 remains in the fossil?
(1) 100%
(2) 50%
(3) 25%
(4) 12.5%
How old is a bone that has 12.5% of the original amount of radioactive carbon-14 remaining?
(1) 5,700 years
(2) 11,400 years
(3) 17,100 years
(4) 22,800 years
The image below shows a spear point embedded in part of a mastodon’s rib bone, found near Seattle, Washington.
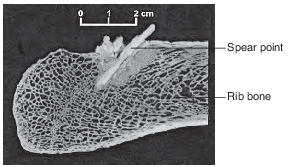
Scientists infer that early North American humans hunted the mastodon. Carbon-14 dating of the rib bone indicates that 2.4 half-lives have passed since the mastodon was killed. Approximately how many years ago did the mastodon die?
(1) 5700
(2) 11,400
(3) 13,700
(4) 17,100
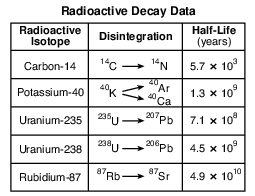
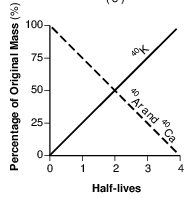
Which graph below represents the amount of potassium-40 and the amount of argon-40 and calcium-40 over four half-lives?
(1) 
(2) 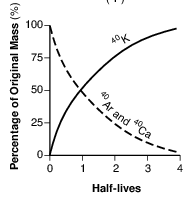
(3) 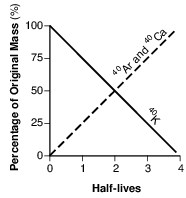
(4) 
Which radioactive isotope is most often used when determining the age of fossil bones found in sediments deposited during the Holocene Epoch?
(1) carbon-14
(2) potassium-40
(3) uranium-238
(4) rubidium-87
Identify the decay product when carbon-14 undergoes radioactive disintegration. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• — 14N
• — nitrogen-14/N-14
• — nitrogen/N
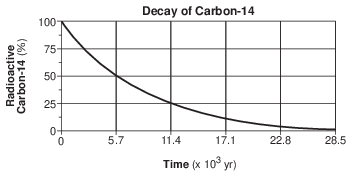
Identify the decay product formed by the disintegration of carbon-14. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• — 14N
• — nitrogen-14
• — N-14
• — nitrogen/N
• — 14C → 14N
A scientist found the bone of a mastodont. In the lab, the scientist found that 12.5% of the original radioactive C-14 still remained in the bone.
Explain why 14C was used to date the mastodont bone. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• — C-14 is used to date recent organic remains.
• — The mastodont bone is less than 50,000 years old.
• — Carbon-14 has a short half-life.
• — Carbon-14 decays at a predictable rate.
State the name of the disintegration product of carbon-14. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• — 14N
• — nitrogen-14
• — N-14

Identify the stable disintegration product that is produced when carbon-14 decays. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• — nitrogen-14
• — 14N
• — 14C → 14N
X The geologic time period when each sedimentary rock layer formed is shown. The symbols ( , , , , and ) represent fossils of different types of organisms present in the rock layers.
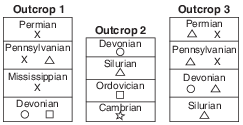
Explain why the geologic age of these rock layers could not be accurately dated using carbon-14. [1]
Allow 1 credit. Acceptable responses include, but are not limited to:
• — Carbon-14 has a short half-life.
• — These rock layers are too old to contain measurable carbon-14.
• — Carbon-14 is used to date recent remains.
• — No organic material remains in the rock.
